Mercury Spill Incidents
- Mercury Spill Incidents is available in Portable Document Format (PDF, 1.05MB, 22pg.)
September 2009
What Do You Know About Mercury?
- Did you play with mercury as a child?
- Do you now know that mercury is toxic and that it can damage the nervous system?
- Do you know that mercury beads can easily cling to items such as shoes, or pets' feet, and be spread to a wider area?
- Do you know that a spill of metallic mercury may require clean up by a professional?
Introduction
This booklet provides information on elemental mercury, its health hazards, and describes an educational campaign about mercury that is taking place in NYS schools. This booklet also provides data on mercury spills reported in NYS from 2000 to 2005, mercury case studies and where to find more information on related topics. The mercury spill data were summarized from the NYS Hazardous Substances Emergency Events Surveillance (HSEES) database.
The appendices at the end of this booklet provide a detailed list of mercury sources, information on interpreting biological sampling for mercury and information on relevant laws, regulations and spill reporting.
Background
Mercury is a naturally-occurring chemical that exists in several forms (metallic, organic and inorganic). Metallic mercury, also called elemental mercury, quicksilver or simply mercury, is the focus of this booklet. It is a shiny, silver-colored metal that is liquid at room temperature. Because mercury has unique properties, it has been widely used in industrial processes, scientific instruments, consumer products and certain ethnic practices.
Mercury is a concern for human health and for the environment. It does not degrade and is not destroyed by burning. In its various forms, mercury cycles through the environment and some forms accumulate in the food chain (for example, as methylmercury in fish). Mercury's toxicity, persistence and widespread use make proper disposal and recycling essential. Familiarity with the uses of mercury can help people locate, manage and replace mercury-containing items.
Mercury Exposure is a Health Concern
Exposure to elemental mercury can occur by breathing mercury vapors, eating or swallowing foods or water contaminated with mercury, or having skin contact with mercury droplets or beads. There is little concern about exposure to liquid mercury from eating or skin contact because the body absorbs little mercury through these routes. The route of exposure that poses the greatest health risk is inhalation of mercury vapor. At room temperature, mercury spills release odorless and colorless mercury vapor into the air where exposure by breathing can occur. Inhaled mercury vapor is readily absorbed from the lungs into the bloodstream, then transported to other parts of the body, including the brain and kidneys.
Air levels of mercury can increase following a mercury spill, particularly in an enclosed space, such as a car or small room. Very small amounts of mercury (for example, a few drops) can raise indoor air concentrations of mercury to levels that may be harmful to health. Since mercury vapor is odorless and colorless, a person can breathe mercury vapor and not be aware that it is entering the body.
Health Effects of Mercury Exposure
Breathing high levels of mercury in air can damage the nervous system and kidneys. Short-term exposure (up to a few weeks) to high levels of mercury can cause cough, shortness of breath, chest pain, nausea, vomiting, diarrhea, fever and hypertension. Longer-term exposure (more than a few weeks) to lower levels can cause tremors, insomnia, irritability, headache and memory loss. Exposure to mercury vapor is of particular concern for children and unborn babies because their nervous systems are still developing and may be more vulnerable. Other potentially vulnerable individuals include those with medical conditions of the nervous system, kidneys, or heart and vascular system. These conditions may be worsened by exposure to mercury. Biological sampling for mercury can serve as a valuable indicator of a person's exposure. Interpretation of biological sampling results is discussed in Appendix 2.
Why Mercury Spills are a Problem
Small spills of mercury (for example, the amount in a fever thermometer) on a smooth, non-porous surface, can be cleaned up safely and easily with proper techniques. However, beads of mercury are heavy and readily sink into cracked floors or other open surfaces. Mercury can be tracked beyond the original spill area on footwear or on pets' feet. Mercury also clings to porous materials like fabric, carpet or wood, making it difficult to remove.
Mercury spill cleanup must be thorough and complete. Inadequate mercury cleaning may lead to long-term exposure from residual droplets or beads. Inadequate cleaning can also increase contamination and cleanup costs. Increased costs can result from replacement of contaminated belongings and from living expenses incurred while the contaminated space is unavailable. Mercury spills that happen indoors where people typically spend long periods of time (such as homes or schools) can be very disruptive and present relocation issues. Relocations from nursing homes, hospitals or prisons are particularly difficult.
Mercury Spills in New York State 2000-2005
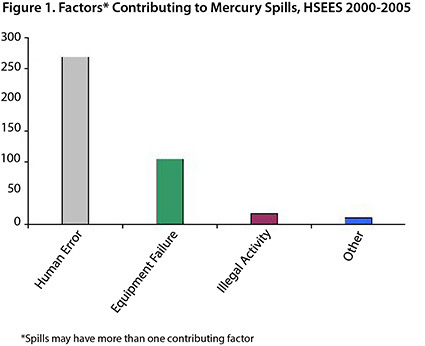
Mercury ranks third (400 spills) among hazardous substances incidents (6,628) that were reported in New York State to the HSEES program from 2000 to 2005. Two-thirds of reported mercury spills (67%) were caused by human error and more than a quarter (26%) were due to equipment failure (Figure 1).
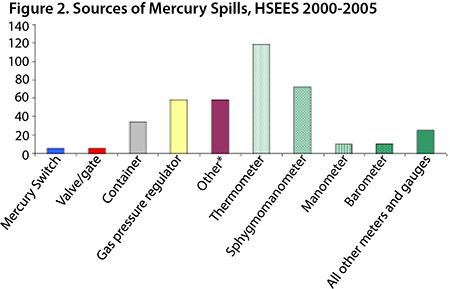
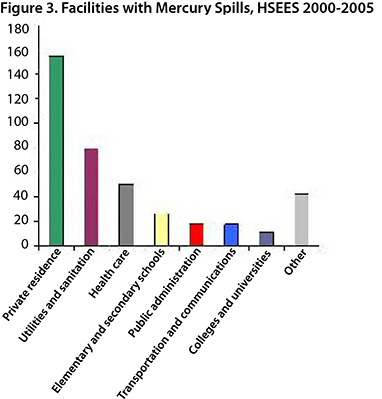
The main sources of reported mercury spills (Figure 2) were meters and gauges (58%) and gas pressure regulators (14%). The mercury spills occurred most frequently in private residences (38%), the utilities and sanitation industries (19%), health care (12%) and elementary and secondary schools (7%) (Figure 3). In recent years, utility companies have conducted programs to replace mercury-containing gas pressure regulators in homes. Installing mercury-free regulators reduces the potential for mercury spills, eliminates the need for mercury cleanup and eliminates the risk of mercury exposure.
Consequences of Mercury Spills
Mercury is a familiar substance to many people. They have seen it in instruments or novelties or, perhaps, even played with mercury beads. Because mercury is so familiar and, unlike many hazardous substances, the vapor has no odor or color that can serve as a warning, a mercury spill may not cause immediate concern and appropriate response. But, a mercury spill must be cleaned up quickly and completely to minimize exposure to toxic mercury vapors. The difficulty in cleaning up a mercury spill depends on the location of the spill, the types of items that become contaminated and if mercury beads are tracked beyond the original spill area. A mercury spill may be readily contained if handled correctly. In contrast, a mercury spill may be readily spread and lead to extensive contamination and disruption if cleanup is delayed or incomplete.
When a mercury spill occurs or is discovered, people should leave the immediate area until it is cleaned to prevent exposure and reduce the risk of potential health effects. Data from the HSEES program show that from 2000 to 2005, 32 reported mercury spills had an evacuation. Half of the evacuations occurred either in homes (8 evacuations) or in schools (8 evacuations).
Because homes and schools had equal numbers of evacuations during this time, it is interesting to compare the impact of the mercury spills in these two settings. The eight mercury spills in private homes caused at least 8 people to be evacuated and the eight mercury spills in NYS elementary and secondary schools caused at least 111 people to be evacuated. It is clear that the mercury spills in schools were disruptive to a greater number of people.
Another way to capture the impact of a mercury spill is to describe the evacuation in person-hours. The term "person-hour" was created as a way to describe the degree of disruption and is calculated by multiplying the number of people evacuated by the number of hours of evacuation. For example, ten people each evacuated for one hour would be ten person-hours; two people each evacuated for five hours would also be ten person-hours. For comparison, the eight evacuations in homes totaled more than 9,780 person-hours whereas the eight evacuations in schools totaled more than 102,294 person-hours. It is clear from the analysis of these spills, that the mercury spills in schools were much more disruptive.
Of the 400 mercury spills reported in New York State from 2000 to 2005...
- 67% were caused by human error
- 58% involved meters and gauges
- 38% occurred in homes
- 25% involved a professional response (HazMat team or contractor)
- 19% occurred in the utilities and sanitation industry
- 9% involved children who were directly responsible for the spill
- 8% involved an evacuation (279 people evacuated)
The HSEES data also show that mercury spills involving children (either causing the spill or spreading the spilled mercury) resulted in the greatest impacts. Mercury spills in homes that involved children accounted for more than two-thirds of the people evacuated from homes (20/28, 71%). Similarly, mercury spills in schools that involved children accounted for the majority of people evacuated from schools (96/111, 86%). Evacuation times which are another measure of disruption can also be compared to gauge whether events with children have a greater impact. Mercury spills in homes in which children were involved accounted for 61% of the evacuation time from homes and 99% of the known evacuation time from schools. Clearly, mercury spills involving children had the most disruptive outcomes whether the spills occurred at home or at school.
Officials reported that six people (two employees and four members of the general public) had health effects or elevated biological sampling results associated with mercury spills. Symptoms included gastrointestinal problems (2) and skin irritation (2). Three people had elevated levels of mercury in their bodies as indicated by biological samples.
What To Do
Facilities that have mercury-containing equipment should catalog those items and develop a spill response policy that includes proper handling of a mercury spill. Staff at each school should be trained to respond appropriately to a mercury spill. Mercury-containing items should be replaced with mercury-free items as much as possible. This can be accomplished over time, either as part of a deliberate phase-out schedule or as the need arises when equipment or appliances need replacement.
Cleanup Guidance
If a mercury spill occurs and you don't know what to do, immediately seek information from someone who does. Professional advice can limit the contamination, prevent exposure, and save time and money. For contact numbers, see the reporting section of this pamphlet or refer to the school brochures listed in the outreach and intervention section.
The NYS Department of Health has developed cleanup guidance for a mercury spill in the home.
Outreach and Intervention
The Partnership to Reduce Mercury in Schools
Mercury and schools are a risky combination because it is easy for even a small mercury spill to contaminate an area and disrupt activities. A series of nine audience-specific brochures was developed to help school personnel identify mercury sources and take steps to reduce the risk of a mercury spill. These educational materials, developed in partnership with representative school personnel, several government agencies and non-profit groups, are intended to provide practical and cost-effective strategies and have been widely distributed to public and private schools in NYS. Also, the NYS Department of Environmental Conservation and the Northeast Waste Management Officials' Association used the brochures in hands-on workshops held statewide to help train school personnel to identify and remove mercury.
Brochures in this series are:
- Mercury and Schools: A Risky Combination (also available in Spanish)
- Reducing Mercury in Schools: Superintendents, Principals, and School Boards
- Reducing Mercury in Schools: Science Teachers
- Reducing Mercury in Schools: Buildings and Grounds Superintendents
- Reducing Mercury in Schools: Health and Safety Committees
- Reducing Mercury in Schools: School Nurses
- Facility-Wide Inventory of Mercury and Mercury-Containing Devices (PDF, 21K, 4pg.)
- Guidelines for Cleanup of Mercury Spills
- Disposal and Recycling Options for Mercury and Mercury-Containing Devices
Case Studies
Case Study #1
The county health department responded to the discovery of mercury beads inside a home, in the garage and on the adjacent sidewalk. Apparently, a plumber working at that location several days earlier had spilled about a 1/4 cup of mercury in the resident's garage. The family was evacuated for two weeks during a contractor's cleanup. Because the family was concerned about possible health effects to the children and pregnant wife, family members went to their private physician for evaluation. One child had elevated levels of mercury in the blood.
Case Study #2
Early one Saturday morning, a floor model sphygmomanometer (blood pressure unit) in the room of a nursing home resident began leaking mercury. The operator rolled the leaking unit down a carpeted hallway to the nurses' station. During the day, staff attempted to clean up the mercury beads in the hallway. Unfortunately, they used a vacuum cleaner which increased the air levels of mercury vapor. Later that day, nursing home staff reported the spill to the Poison Control Center, who then contacted the local health department. The county health department, several fire departments, the county HazMat team, law enforcement, and almost a dozen ambulances responded to the nursing home. During that night and the next day, 32 nursing home residents were evacuated to area hospitals. Some residents remained evacuated for several days while a contractor cleaned up the mercury contamination. Mercury was found in the original spill areas and in other areas where it had been spread by shoes or wheels.
Case Study #3
During a successful day at the flea market, one homeowner purchased an antique mercury barometer and brought it home in the trunk of the family car. The homeowner later discovered spilled mercury in the vehicle (parked in the garage) and in at least two rooms of the home. The resident hired a consultant to identify all areas of mercury contamination and to clean the home, the vehicle and the barometer. After cleanup, air sampling showed that the vehicle was still contaminated by mercury. After a piece of contaminated carpet was removed and the car was ventilated, air sampling showed that cleanup was complete. The residents reported no health effects, but were referred to an occupational health clinic to address their health concerns.
Case Study #4
One chilly winter afternoon, a home remodeler was using a plumber's manometer to measure gas pressure in his kitchen's gas line. Gas pressure from the line shot the mercury out onto the rough plywood floor and behind the refrigerator. After vacuuming most of the mercury with a shop vacuum, the homeowner called both the Local and State Health Departments for guidance. He then learned that vacuuming increases the amount of mercury in the air and that a vacuum cleaner contaminated by mercury is hazardous waste. The contaminated shop vacuum was double-bagged until it could be discarded at the town's hazardous waste disposal facility. The area of the spill in the kitchen could not easily be isolated because the kitchen was being remodeled and the wall separating the kitchen from the living room had been removed. The homeowner hired an environmental firm to clean up the mercury and conduct air sampling to verify that cleanup was completed. Meanwhile, his children and pregnant wife stayed with relatives to avoid exposure to mercury vapor.
Case Study #5
A science teacher arrived at school to prepare for the coming school year and noticed that a mercury bottle had spilled in the chemical storage closet. The spill had not been previously reported and was apparently accidental. As school staff attempted to clean up the spill with a mercury spill clean-up kit, they found more areas with spilled mercury. Hazardous materials contractors, using instruments to measure air levels, located mercury in a few other areas of the school. No health effects were reported and access to contaminated areas was restricted until cleanup was completed. The State Health Department assisted the school community by addressing health concerns expressed by parents and others. Clean-up costs for this mercury spill exceeded $22,000.
Case Study #6
Two students broke a blood pressure machine in a school nurse's office, spilling mercury on themselves and on a nearby shelf. School staff restricted access to the room and called HazMat and the local health department who responded immediately. Air measurements near the students' clothing indicated that the clothes were contaminated with mercury. The students were instructed to shower and change clothes. Their clothing was sealed in plastic bags for proper disposal by a hazardous waste contractor, who also cleaned the nurse's office. Officials' were concerned about the nearness of the school cafeteria to the spill site, but air sampling revealed that the air in the cafeteria was not contaminated.
Case Study #7
One Tuesday turned out to be anything but ordinary when secondary school staff was notified that some 6th graders had located a small bottle of mercury. The mercury, in a closet for many years, had been spilled in the science classroom. At least 20 people were temporarily evacuated from that section of the building as a precaution, and a dozen students required decontamination. The school consulted with a Poison Control Center, the local health department, Board of Cooperative Educational Services (BOCES), New York State Department of Health, and others about clean-up issues and health concerns. Room access was restricted through the weekend during cleanup, but the room was re-opened on Monday morning.
Case Study #8
One day, a family was surprised to find tiny, silvery beads dripping from a doorjamb. They investigated the mysterious drops and discovered that the source was a container of mercury, one of several long-forgotten items stored in their attic. When the container broke, about a pound of mercury seeped through the attic floor into the room below. A HazMat team responded. Two people had to evacuate the residence for approximately nine days until cleanup was complete.
Case Study #9
A wall-mounted blood pressure unit in a doctor's office examination room failed spilling mercury on the tiled floor. The facility closed the room and hired a contractor to clean up the mercury. After cleanup, air sampling results indicated that mercury was still present. When facility staff called the State Health Department for advice, the Department conducted further air sampling and located additional beads of mercury. In all, it took the contractor several days and three cleaning cycles to completely remove all the mercury and decontaminate the office.
About HSEES
HSEES was a state-based, federal program that collected and analyzed data on incidents involving spills or air releases of non-petroleum hazardous substances. The goal was to reduce morbidity (injury) and mortality (death) resulting from hazardous substance emergency events by identifying risk factors in the incident data. In 2010, the National Toxic Substance Incidents Program replaced the Hazardous Substances Emergency Events Surveillance which was established in 1990 by the Agency for Toxic Substances and Disease Registry.
This mercury booklet was produced by New York HSEES staff to protect human health and the environment by providing information and education to prevent future spills. Understanding the causes, sharing the lessons learned and integrating these lessons into activities such as planning, training and maintenance can be a major part of prevention.
Additional Resources
There is much information available on mercury. Two good resources are:
ToxFAQs for Mercury CAS#743 9-97-6. 2011. Agency for Toxic Substances and Disease Registry. US Department of Health and Human Services, Atlanta, GA.
Toxicological Profile for Mercury. 2011. Agency for Toxic Substances and Disease Registry. US Department of Health and Human Services, Atlanta, GA.
Appendix 1 - Sources of Mercury
Mercury has been widely used in industrial processes, scientific instruments, consumer products and certain ethnic practices. Familiarity with these uses can help people locate, manage and replace mercury-containing items. The table below lists examples of areas and items that may contain mercury:
| Locations and Uses of Mercury | Examples |
|---|---|
Industrial/Commercial Uses1
|
|
Mechanical/Electrical Systems and Plumbing
|
|
| Scientific Instruments3 |
|
Schools
|
|
Health Care Industry
|
|
| Consumer Products |
|
| Cultural and Religious Practices5 |
|
| Residences | Most of the specific examples listed in this table can also apply to living space. Consider how the home was built or remodeled and the different activities that have occurred. |
Notes:
- Abandoned manufacturing facilities may have containers of mercury on site. Even a small amount of mercury can cause extensive contamination, especially if tracked or carried off-site.
- Mercury is 13 times heavier than water and remains in the bottom of u-shaped traps or other low areas. Water covers the mercury and prevents evaporation when the sink is in use, but unused sinks can dry out and allow mercury vapors to escape to the indoor air. Any repair work that involves opening pipes or traps should be done carefully to avoid a spill if mercury is present.
- These instruments measure properties such as temperature, pressure, vacuum, humidity, flow and wind speed.
- Dental offices may be contaminated from past spills of elemental mercury during preparation of dental amalgams.
- Mercury can be purchased under the name "azogue" in stores (called botanicas) that specialize in religious items associated with Santeria, Espiritismo, and Voodoo practices. The U.S. Environmental Protection Agency's (EPA) Office of Emergency and Remedial Response (OERR) convened a Task Force on Ritualistic Uses of Mercury in January 1999 to gain a better understanding of these practices and their potential public health impacts. For more information
Appendix 2 - Interpretation of Biological Sampling Results
After a person is exposed to a hazardous substance, it is sometimes possible to measure that substance in a biological sample such as the blood or the urine. These biological sampling results can be valuable because they indicate the degree of a person's exposure. Biological sampling results can also serve as a valuable indicator of decreasing levels of the hazardous substance in the body. The levels can decrease either because of the natural clearing processes of the body over time, because of medical treatments, or because of both time and treatments. Staff at occupational health clinics can help primary care physicians interpret biological sampling results for mercury from adults or children. They have more experience, typically from patients with workplace exposures, than most health care providers. New York State Department of Health has a list of occupational health clinic locations and contact numbers in NYS.
Pediatricians can help parents with questions about biological sampling if children are exposed to mercury. If sampling is indicated, pediatric environmental health physicians and occupational health clinics can help interpret the results. Children who have had mercury poisoning may require periodic neurological examinations for follow-up.
Appendix 3 - Reporting and Relevant Laws/Regulations
Mercury spills of one pound or more (approximately two tablespoons) must be reported within two hours (40 CFR Part 302; 6 NYCRR Parts 595 and 597) to:
- National Response Center (NRC) at 1-800-424-8802 and
- NYS Department of Environmental Conservation Hotline at 1-800-457-7362.
Mercury spills in New York City schools should also be reported to:
- New York City Department of Environmental Protection's Division of Emergency Response & Technical Assessment (DERTA) at 718-595-4646. If that line is busy, call 9-1-1 and ask to be connected to DERTA.
- New York City Department of Education, Office of Environmental Health and Safety at 718-361-3808.
New York State Heavy Metals Registry
The NYS Heavy Metals Registry is a monitoring program established by the NYS Department of Health under the State Sanitary Code. Every doctor, clinical laboratory and health facility in NYS is required to report elevated levels of mercury in blood or urine to the NYS Department of Health within ten days. An elevated mercury level is a test result that is greater than or equal to 5 nanograms mercury per milliliter of blood, or 0 nanograms mercury per milliliter of urine. More information about the NYS Heavy Metals Registry.
New York State Mercury-Added Consumer Products Law
The Mercury-Added Consumer Products Law of 004 (amended in 005) includes provisions about labeling, usage, disposal and recycling of mercury-added consumer products (such as scientific instruments with mercury) and bans mercury-added novelty consumer products (such as toys, games, ornaments and footwear). This law also prohibits the use of mercury in all primary and secondary schools in NYS. Further information is available at the NYS Department of Environmental Conservation's web site.
Dental Mercury
Dentists have traditionally filled cavities with small quantities of mercury mixed with silver and other metals to create an amalgam. NYS law now requires that dentists use encapsulated mercury and recycle any mercury or dental amalgam waste generated in their offices. For more information on this topic.
Mercury in Vehicles
Information on the New York State law to properly manage mercury-containing switches in automobiles.