Medicaid Drug Cap Webinar
Jason A. Helgerson, Medicaid Director
New York State Department of Health
- Presentation also available in Portable Document Format (PDF)
August 31st 2017
Agenda
- Overview of Medicaid Drug Cap Statutory Provisions
- SFY 17–18 Drug Cap Baseline Calculation
- SFY 17–18 Drug Cap Projection
- Identification of Drugs for Possible Drug Utilization Review Board (DURB) Referral
- Negotiation Process
- Period Before Drugs Are Referred to the DURB
- Drugs Referred to the DURB
- DURB Evaluation & Recommendation
- After DURB Recommendation
- DURB Purpose and Enhanced Authority
- Next Steps
Overview of Statutory Provisions – Section 280 PHL
Establish Rebates for High Cost Drugs ($55 million State Share savings in 2017–18)
- Limits drug spending growth in SFY 2018 to the 10–year rolling average of the medical component of the Consumer Price Index plus five percent, less the State share rebate target of $55 million;
- Authorizes the Department of Health (DOH) to negotiate enhanced rebates with drug manufacturers in the event that the Director of the Budget determines drug spending is projected to exceed the Cap;
- Authorizes the Commissioner to refer certain drugs to the DURB;
- Augments the DURB membership to include two health care economists, one actuary and a representative from the Department of Financial Services (DFS);
- Authorizes the DURB to request drug development, cost/pricing, and other data to determine appropriate target rebate amount; and
- Authorizes the Commissioner to require prior authorization, directing Medicaid Managed Care (MMC) plans to remove drug(s) from their formularies, waiving prescriber prevails provisions and accelerating rebate collections.
Drug Cap Target Calculation (Baseline)
- The SFY 2017–18 Budget sets a baseline drug spending target utilizing SFY 2016–17 managed care and fee–for–service (FFS) drug expenditures and rebates trended by the ten–year rolling average of the medical component of the Consumer Price Index (CPI) (3.2% at enacted) plus five percent.
- The Cap is then reduced by the SFY 2017–18 pharmacy savings target of $55M State share (growing to $85M State share in SFY 2018–19).
SFY 17–18 Drug Cap Projection
- Pursuant to Section 280 of the Public Health Law, the Division of the Budget (DOB) and the Department of Health analyzed the projected SFY 2017–18 State Medicaid drug spending.
- DOB concluded that expenditures are projected to exceed the Medicaid Drug Cap.
- Drug spending is projected to exceed the Drug Cap by $119M State share. This is driven by an overall 15% year–to–year increase in managed care and FFS State share net pharmacy spend.
- The managed care expenditures which drive a majority of the increase (18%) was calculated by the State´s actuary (Mercer).
SFY 2017–18 Medicaid Drug Cap
1. Program FY 2017 Base 2. FY 2018 Projection MMC $ 4,045,404,620 $ 4,739,842,990 HARP $ 350,846,820 $ 544,982,978 HIV SNP $ 520,391,466 $ 528,065,007 FFS $ 745,929,124 $ 723,551,250 Gross Pharmacy Spend $ 5,662,572,030 $ 6,536,442,225 3. State Portion of Gross Managed Care Rx Spend $ 1,912,574,091 $ 2,261,214,589 3. State Portion of FFS Rx Spend $ 332,684,389 $ 322,703,858 Total State Rx Spend $ 2,245,258,480 $ 2,583,918,447 4. OBRA and State Supp. Rebate Adjustment $ (1,309,698,742) $ (1,507,245,054) % of Total State Rx Spend 58.3% 58.3% Net Baseline Medicaid Drug Spend $ 935,559,738 $ 1,076,673,393 10 year Avg. Annual CPI Medical Trend + 5.0% 8.2% N/A Pharmacy Savings Target Adjustment $ (55,000,000) N/A SFY 2017–18 Medicaid Drug Cap $ 957,640,505 N/A Projected Excess/(Shortfall) $ 119,032,888 Estimated Target for Additional Rebates % 1.8%
- Managed Care programs and/or premiums with less than $10M in pharmacy spending were excluded.
- Managed Care projected Total Cost calculated by multiplying projected Members Months by projected Per Member Per Month (PMPM) premium. FFS projected Total Cost based on SFY 2017–18 global cap projections and also includes any pharmacy products currently "carved out" of Managed Care. FFS and Managed Care Total Cost excludes physician administered drugs.
- Assumes 38.9% State Share allocation for Managed Care and 44.6% State Share allocation for FFS consistent with SFY 2016–17 actual experience.
- Assumes the same weighted State Share SFY 16–17 spend allocation for Managed Care and FFS combined.
Identification of Drugs for DURB Referral
- Under the statute, if it is determined that projected expenditures will exceed the annual growth limitation imposed by the Drug Cap, the Commissioner may refer drugs to the DURB for recommended target supplemental rebates.
- In making DURB referrals, the DOH considers the State´s cost (including rebate amounts), and whether the manufacturer is providing significant discounts relative to other drugs covered by the Medicaid program.
- DURB recommended target rebate amounts:
- The Commissioner cannot require a rebate which is greater than the DURB recommended target rebate
- Rebates are effective April 1, 2017
Process for Initial Identification of Drugs for Possible DURB Referral (Visual Flowchart)
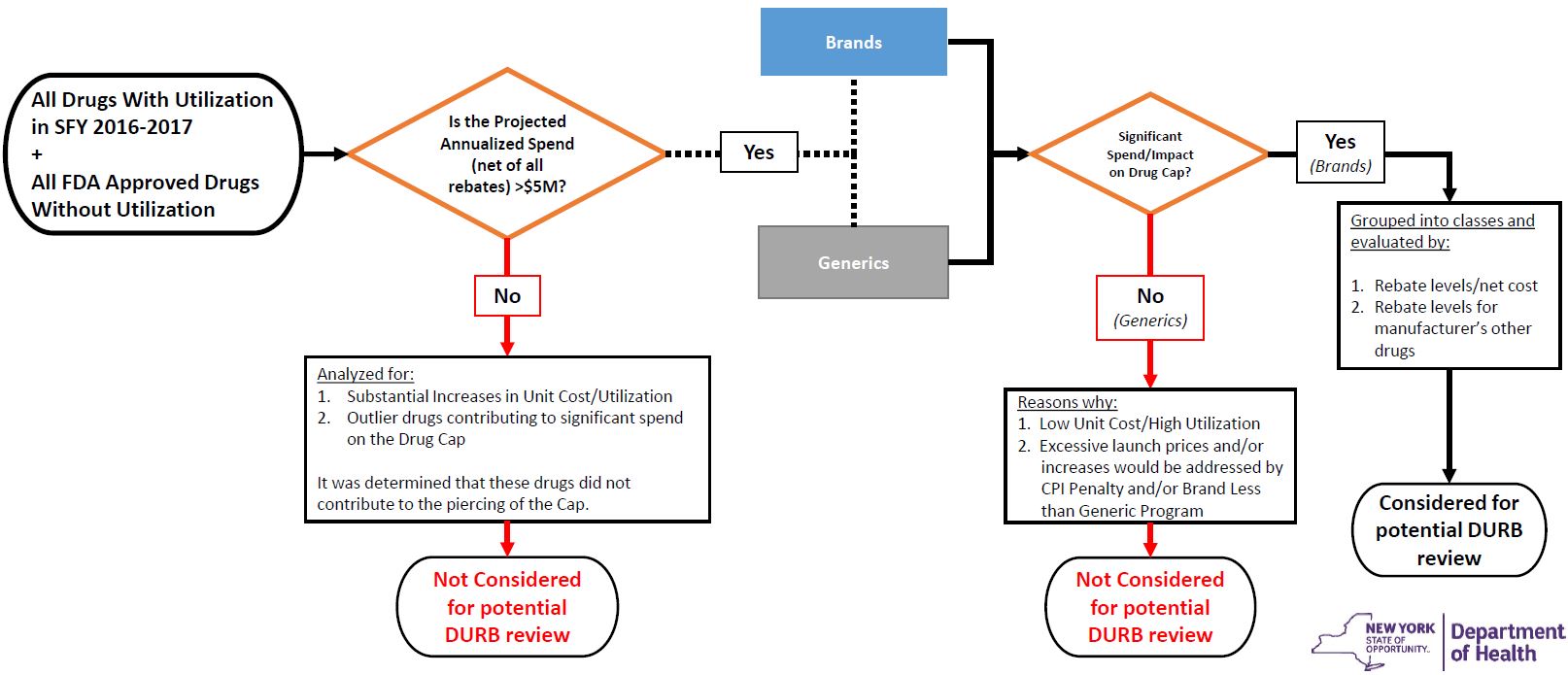
Process for Initial Identification of Drugs for Possible DURB Referral (Figures)
| Category | All drugs with utilization in SFY 2016–2017 + All drugs without utilization in SFY 2016–2017 |
Drugs with projected SFY 2017–2018 spend >$5M net of all rebates |
Identification of Drugs for Possible DURB Referral |
|---|---|---|---|
| Number of Drugs | 7,662 | 73 | 30 |
| Number of Manufacturers | 594 | 70 | 12 |
| Percent of Spend net of all rebates (State Share) |
100% | 51% | 37% |
Negotiation Process
Step 1: Period Before Drugs Are Referred to the DURB
- Pursuant to Section 280 of the Public Health Law, if DOH intends to refer a drug to the DURB – the department will notify the manufacturer of such drug.
- This notification was sent to affected Drug Manufacturers on 08/25/2017.
- DOH will attempt to reach agreement with manufacturers on a rebate for the drug(s) prior to referring the drug(s) to the DURB for review.
Step 2: Drugs Referred to the DURB
- PHL Section 280, authorizes DOH to refer a drug to the (DURB) for a recommended target supplemental rebate amount.
- 30 days prior to DURB meeting, DOH will post an agenda on its website, which will include the names of drugs to be reviewed by the DURB for recommended target supplemental rebate amounts.
- Until such time that the DURB meets, manufacturers and the DOH may continue to work towards an agreement regarding supplemental rebate amounts.
Step 3(a): DURB Evaluation
In determining whether to recommend a target supplemental rebate for a drug the DURB may consider:
- The cost of the drug to the NYS Medicaid program (including Federal/State rebates);
- The drug´s impact on the Medicaid drug spending growth target and the adequacy of capitation rates of participating MMC plans, and the drug´s affordability and value to the Medicaid program;
- Significant and unjustified increases in the price of the drug; and
- Whether the drug may be priced disproportionally to its therapeutic benefits.
Step 3 (b): DURB Recommendation
In formulating a recommendation, the DURB may consider:
- Publicly available and DOH supplied pricing information and information related to value–based pricing;
- The seriousness and prevalence of the disease or condition being treated;
- Medicaid utilization and the drug´s effectiveness or impact on improving health, life quality or outcomes;
- The likelihood that the drug will reduce the need for other medical care, including hospitalization;
- The average wholesale price, wholesale acquisition cost, retail price, and cost of the drug to Medicaid minus rebates;
- Whether there are pharmaceutical equivalents to the drug; and
- Information provided by the manufacturer, if any, regarding pricing and development costs, therapeutic benefits and/or other information pertinent to pricing decisions, which shall be considered confidential.
Step 4: After DURB Recommendation
- Pursuant to PHL Section 280 – If after the DURB recommends a target rebate amount, DOH and the manufacturer still have not reached agreement regarding supplemental rebate amounts, the manufacturer will be required to provide DOH with information related to the actual costs of developing, marketing, researching and distributing the drug.
- PHL Section 280 also authorizes:
- Directing managed care plans to remove from their Medicaid formularies those drugs for which a manufacturer has failed to enter into a rebate agreement;
- Subjecting drugs to prior approval;
- Promoting the use of cost effective and clinically appropriate drugs other than those of a manufacturer who has failed to enter into a rebate agreement; and
- Accelerating rebate collections.
Drug Utilization Review Board (DURB)
Overview and Process
Purpose
The DURB provides clinical guidance to the Commissioner regarding the utilization of pharmaceuticals and the evaluation of drug expenditures within the Medicaid program.*
DURB activities include but are not limited to the following:
- Establishment and implementation of medical standards and criteria for the prospective and retrospective DUR program;
- Development, selection, application, and assessment of educational interventions for physicians, pharmacists and recipients that improve care;
- Administration of pharmacy programs including the Preferred Drug Program and the Clinical Drug Review Program; and
- Evaluation of drugs which that contribute to exceeding the Drug Cap and making recommendations for target supplemental rebates (New).
*The purpose and responsibilities of the DURB are established in Social Services Law section 369–bb and Public Health Law Title 1 sections 270, 272, 274 and 280.
DURB Membership
DURB members are appointed by the Commissioner and the Board consists of twenty–three members:
- One chairperson representing the Department of Health;
- Six licensed and actively practicing physicians;
- Six licensed and actively practicing pharmacists;
- One licensed and actively practicing nurse practitioner or midwife;
- Two drug utilization review experts, at least one of whom is a pharmacologist;
- Three consumers or consumer representatives;
- Two health care economists (New);
- One actuary (New); and
- One representative from the NYS Department of Financial Services (DFS) (New).
DURB Process
Drug Reviews will be integrated into the current DURB processes:
- Publicly Held Meeting
- Agenda Posted 30 days prior to Meeting
- Public Comment Period
- Financial Information Reviewed in Executive Session
- DURB Recommendations Announced at Meeting
- DURB Recommendations Posted after Meeting
- 5 Day comment Period
- Commissioner Final Determinations Posted
Next Steps
| Activity | Target Date(s) |
|---|---|
| DOH/Manufacturer Negotiations | Immediately |
| DURB Referral | 30 days prior to the DURB meeting, DOH will post an agenda on its website, which will include the names of drugs that will be reviewed for recommended target supplemental rebate amounts |
| DURB Meeting(s) | October December |
Questions?
- Questions should be sent to MADrugCap@health.ny.gov.
- Frequently Asked Questions (FAQs) will be posted, with notification via the MRT listserv.
Follow Us