New York State Medicaid Update - April 2024 Volume 40 - Number 4
In this issue …
- Policy and Billing Guidance
- New York State Medicaid Billing Guidelines for Administering Mental Health or Substance Use Disorder Treatment Injections at the Pharmacy and Record Documentation and Retention
- New York State Medicaid Fee-for-Service Covers Adult Vaccinations Without Cost-Sharing
- Practitioner Administered Drug Update: Brixadi™ Billing Guidance
- Reminder: Compound Policy
- NYRx Coverage Policy and Billing Guidance for Pharmacist Dispensing of Self-Administered Hormonal Contraception
- New Medicaid Enrollment Option for Non-Enrolled Providers Seeking Medicare Bad Debt
New York State Medicaid Billing Guidelines for Administering Mental Health or Substance Use Disorder Treatment Injections at the Pharmacy and Record Documentation and Retention
Effective April 1, 2024, in accordance with the amendments to §6801 and §6802 of Chapter 802 of the Laws of 2022, registered pharmacists, certified by the New York State (NYS) Education Department (ED) may administer a long-acting injectable (LAI) approved by the Food and Drug Administration (FDA) for the treatment of mental health or substance use disorder based on a patient specific prescription or order after the initial injection was provided in the office of the practitioner.
Policy:
- The certified pharmacist must confirm the initial injection has been given by the practitioner and the NYS Medicaid member is ready for maintenance therapy.
- The NYS Medicaid member or the legally responsible representative of the NYS Medicaid member must consent to the drug administration by a certified pharmacist.
- The pharmacy must provide a private area for the drug administration. Ideally, the area should have seating, constructed to maximize auditory and visual privacy, and minimize barriers to communication.
- The certified pharmacist must notify the prescriber of the NYS Medicaid member of the drug administration, and if there were adverse reactions or any side effects.
- Notification must take place within five days via an interoperable electronic medical records system, electronic prescribing technology or pharmacy record, or other electronic transmission or facsimile. If electronic means are not available, the information may be communicated by telephone.
Documentation
The following information must be documented and retained in the record of the pharmacy:
- Contemporaneous records evidencing consent by NYS Medicaid member or representative to drug administration by a certified pharmacist.
- Confirmation that the initial injection has been given by the practitioner and the NYS Medicaid member is ready for maintenance therapy.
- Date and time of the administration by the pharmacist.
Billing Guidelines
Pharmacies will be reimbursed for the medication and an administration fee when billed to NYRx, the NYS Medicaid Pharmacy program. The NYS Medicaid member will not have a copayment for drug administration. NYS legislation requires a patient specific prescription or order for administration. These orders must be kept on file at the pharmacy. If billing for both the drug and administration, these claims must be submitted separately.
For the drug, the claim must be submitted using the National Provider Identifier (NPI) of the ordering prescriber in the Prescriber ID field. The pharmacy will be reimbursed for the medication when billed via National Drug Code (NDC) using reimbursement methodology. For the administration, the claim must be submitted using procedure code "96372" and the pharmacy NPI in the Prescriber ID field. A pharmacy will be reimbursed the administration fee of $13.36.
Questions and Additional Information:
- Pharmacists should refer to the NYSED "Pharmacists" web page, for additional information.
- NYRx claim questions should be directed to the eMedNY Call Center at (800) 343-9000.
- NYRx Pharmacy coverage and policy questions should be directed to the NYS Medicaid Pharmacy Policy Unit by telephone at (518) 486-3209 or by email at NYRx@health.ny.gov.
New York State Medicaid Fee-for-Service Covers Adult Vaccinations Without Cost-Sharing
New York State (NYS) Medicaid covers vaccines and their administration, without cost-sharing obligations, for any outpatient clinic visit in which an approved adult vaccine, recommended by the Advisory Committee on Immunization Practices (ACIP), was administered as part of the visit.
Billing Instructions for Fee-for-Service
The eMedNY system has been modified to systematically bypass all NYS Medicaid member cost-sharing requirements for any outpatient clinic visits in which an approved adult vaccine, recommended by the ACIP, was administered and no payment reduction will occur. Clinics no longer need to append modifier "33" to the applicable Current Procedural Terminology (CPT)/Healthcare Common Procedure Coding System (HCPCS) code for the ACIP-recommended vaccine when submitting an outpatient Ambulatory Patient Group (APG) or Ordered Ambulatory claim to NYS Medicaid.
Questions and Additional Information:
- Fee-for-service (FFS) billing and claim questions should be directed to the eMedNY Call Center at (800) 343-9000.
- NYS Medicaid FFS medical coverage and policy questions should be directed to the Office of Health Insurance Programs (OHIP) Division of Program Development and Management (DPDM) by telephone at (518) 473-2160 or by email at FFSMedicaidPolicy@health.ny.gov.
Practitioner Administered Drug Update: Brixadi™ Billing Guidance
Effective April 1, 2024, for New York State (NYS) Medicaid fee-for-service (FFS), practitioners must submit a claim for Brixadi™ with a unit of "1" when the drug is administered in a medical office. The Centers for Medicare and Medicaid Services (CMS) has categorized Brixadi™ with reimbursement rates by the unit, not milligrams or milliliters. A set rate has been established for the Healthcare Common Procedure Coding System (HCPCS) codes below:
- "J0577" – "Injection, buprenorphine extended-release (Brixadi™), less than or equal to seven days of therapy."
- "J0578" – "Injection, buprenorphine extended-release (Brixadi™), greater than seven days and up to 28 days of therapy."
Billing Units Clarification
Providers should refer to the examples provided below to assist in completing the electronic Provider Assisted Claim Entry System (ePACES) electronic form or the Medical Assistance Health Insurance Claim Form (eMedNY 150003 form). Please note: When completing sections pertaining to National Drug Code (NDC), all formulations of Brixadi™ must be submitted with a value of "1" as the HCPCS unit quantity and the volumetric quantity administered in milliliters.
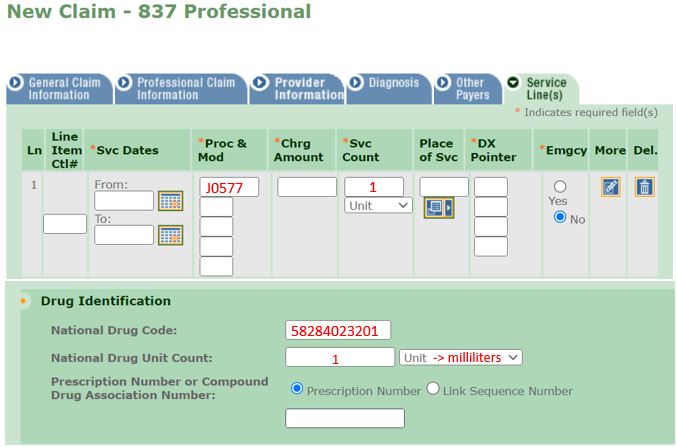
Electronic Claim Example for Brixadi™ Weekly 32mg/0.64ml Syringe
150003 Paper Claim Example for Brixadi™ Weekly 32mg/0.64ml Syringe
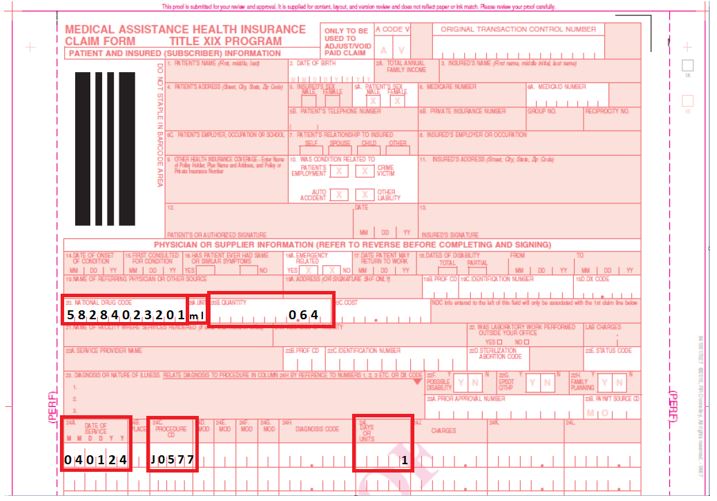
When completing the Medical Assistance Health Insurance Claim Form (eMedNY 150003 form) for Brixadi™, providers should refer to the following instructions:
- Field 20: National Drug Code
The 11-digit unique identifier of the drug administered. - Field 20A: Unit
The NDC unit of measure. All claims for Brixadi™ will be entered as milliliters or "ml". - Field 20B: Quantity
Enter the volumetric quantity in milliliters in this field. - 24C: Procedure Code
The primary five-digit HCPCS code. - 24I: Days or Units
Enter a value of "1" for all Brixadi™ claims.
Questions and Additional Information:
- Practitioners should refer to the ePACES Professional Real Time Claim Reference Guide, for additional information.
- FFS billing and claim questions should be directed to the eMedNY Call Center at (800) 343-9000.
- FFS provider billing guidance can be found in the eMedNY New York State 150003 Billing Guidelines – Physicians and in the Policy and Billing Guidance Ambulatory Patient Groups (APGs) Provider Manual.
- Practitioners should refer to the Clarification of Previous Guidance: New York State Medicaid Fee-for-Service Coverage of Practitioner Administered Drugs article published in the July 2022 issue of the Medicaid Update, for additional information.
Reminder: Compound Policy
This guidance is a reminder for pharmacies regarding compound prescription medications billed to New York State (NYS) Medicaid. Pharmacies must follow the compound policy as updated and previously communicated in the following Medicaid Update articles:
- Compound Coverage Clarification article published in the August 2022 issue of the Medicaid Update;
- Update on Pharmacy Billing for Compound Prescriptions article published in the March 2022 issue of the Medicaid Update;
- Compound Policy: A Reminder and Clarification article published in the December 2020 issue of the Medicaid Update; and
- Update on Medicaid Fee-for-Service Prior Authorization of Topical Compounded Drug Products article published in the November 2018 issue of the Medicaid Update.
NYS Medicaid Compound Policy
NYS Medicaid acknowledges the need for traditional extemporaneous compounding to customize a drug prescribed for a NYS Medicaid covered medically accepted indication which the therapeutic amounts, combinations, and route of administration are Federal Drug Bureau (FDA) approved or compendia supported and there are no suitable commercially available products within the drug class. The Medicaid compound policy is as follows:
- Only the dispensing pharmacy may prepare the prescribed compounded prescription.
- Dispensing outsourced prepared compounds is not allowable.
- Compounds trademarked by pharmacies are not coverable.
- Refills of compounds must be specifically requested by the patient or the authorized agent of the patient before the item is prepared and submitted for payment.
- Food and Drug Administration (FDA)-approved ingredients; or ingredients on the 503A FDA bulk list under Category 1, submitted on compound claim; must be available on the eMedNY "Medicaid Pharmacy List of Reimbursable Drugs" web page.
- Compounds may not contain excluded drugs or be made for NYS Medicaid excluded indications as per the Social Security Act §1927(d)(2), including, but not limited to drugs to treat weight loss or sexual dysfunction or for cosmetic purposes.
- Compounds may not be made to bypass the criteria within the NYRx, the Medicaid Pharmacy Program Preferred Drug List. Compounds may not be made with or to replace drug products removed from the marketplace due to safety reasons.
- Compounds may not be made in therapeutic amounts or combinations not FDA-approved or compendia-supported for use.
- Compounds may not be made to add coloring, flavoring, perfumes, or other non-active ingredient additives to a commercially available product.
- Submitted compound claims may not include packaging materials or containers, syringes, or other items utilized or necessary in the preparation or use of final compounded product.
- Compounds must be adjudicated with the appropriate and matching final compounded product route code for the dosage form.
- Prepared compounds that mimic a commercial product must include on the prescription and in the members medical chart documentation of the reason for compounding (i.e., sensitivity or contraindication to dyes, preservatives, or fillers or lack of availability of a commercial product).
- The FDA-approved or compendia-supported use and dose of an ingredient must match the compound´s intended therapeutic use.
- Compounding kits packaged for convenience with premeasured ingredients are not covered as an outpatient drug per Social Security Act §1927(k)(2)(A)(i) and Social Security Act §1902(a)(54). Providers should refer to the billing section provided below for claim submission of individual ingredients.
- Reconstitution per product labeling is not considered a compound whether it comes as a kit or requires additional supplies, including those prepared for topical, oral, or parenteral use.
- All NYS Medicaid policies, as well as NYS and federal laws, rules and regulations apply.
The NYS Department of Health (DOH) has made and will continually make formulary updates and system enhancements to support this policy. The Office of the Medicaid Inspector General (OMIG) will continue to review claims for policy adherence.
Topical
Examples of non-covered topical compound claim submissions are those made with ingredients which are not FDA-approved, compendia-supported or excluded from NYS Medicaid coverage for topical use, including but not limited to:
- anticonvulsants;
- non-steroidal anti-inflammatory drugs (NSAIDS);
- skeletal muscle relaxants;
- combinations of two or more antifungals;
- foot baths or soaks;
- other soaks; or
- irrigations.
Oral
Examples of non-covered oral compound claim submissions are those made:
- with ingredients not FDA-approved, compendia-supported for oral use or excluded from NYS Medicaid coverage; or
- by reconstituting commercially available products; or
- as an enteral nutrition product.
Providers can refer to the correct billing guidance for these products in the eMedNY Medical Supplies Procedure Codes and Coverage Guidelines.
Parenteral
Examples of non-acceptable parenteral compound claim submissions are those:
- inconsistent with sterile compound criteria as required by State or federal law or regulation [Title 8 of the New York Codes, Rules and Regulations (NYCRR) Part 29.2 (13)], or
- made to simply dilute, reconstitute, or otherwise prepare a medication for infusion per its labeling, or
- products made for nutrition or hydration.
Providers can refer to the correct billing guidance for these products in the eMedNY Medical Supplies Procedure Codes and Coverage Guidelines.
Pharmacy Billing
Per NYS Medicaid policy, when billing a compound via National Council for Prescription Drug Programs (NCPDP) D.0 transaction, providers must submit a minimum of two ingredients [or National Drug Codes (NDCs)] in the Compound Segment, field 489-TE (Compound Product ID). Providers can submit up to 25 NDCs using this field. Providers must also submit a compound code of "02-Compound" in field 406-D6 (Compound Code) in the Claim Segment. Claims with NDCs listed in the Compound Segment submitted as a compound code "01-Not a compound" will not be accepted. All compound claims must include a valid Route of Administration code using terminology from the Systematized Nomenclature of Medicine–Clinical Terms (SNOMED–CT) in NCPDP field 995-E2. All ingredients of a compounded prescription must be submitted to NYS Medicaid regardless of reimbursement.
| eMedNY Edit Number/Message | NCPDP Response Code/Description |
|---|---|
| 70420 – Compound Segment found when compound drug code is not compound | 8D – Compound Segment present on a non-compound claim |
Compound-Only Ingredients
Items intended for compound use only, such as suspending agents or bulk powders, may only be reimbursed as part of a compound claim. These items will not be reimbursed when submitted as a single ingredient claim and will deny when compound code "01-Not a compound" is submitted in field 406-D6 (Compound Code) in the claim segment.
| eMedNY Edit Number/Message | NCPDP Response Code/Description |
|---|---|
| 02326 – Drug only covered in compound | 8D – Product/service only covered on compound claim |
Non-Reimbursable Ingredients
Payment will only be issued for drugs found on the eMedNY "Medicaid Pharmacy List of Reimbursable Drugs" web page. If an ineligible drug or drug product is included in the compound, the claim will deny. The pharmacy provider may then elect to receive payment only for those reimbursable drugs by resubmitting the claim with of "08-Process Compound for Approved Ingredients" in field 420-DK (Submission Clarification Code) when all other coverage criteria is met. Please note: Submission Clarification Code of "08" should only be utilized in accordance with NYS Medicaid policy. The NYS DOH will monitor the use of these codes.
Prior Authorization
Per the September 2015 NYS Medicaid Drug Utilization Review (DUR) Board recommendations, and the Update on Medicaid Fee-for-Service Prior Authorization of Topical Compounded Drug Products article published in the November 2018 issue of the Medicaid Update, prior authorization (PA) and editing on prescriptions for all topical compounded drug products is required and will be implemented on certain NYRx Medicaid fee-for-service (FFS) claims. This process will ensure that compounded topical drug products meet State and Federal regulations and that the compound ingredients are FDA-approved or compendia-supported for topical use when submitted to NYRx.
Pharmacies that submit topical compounds may receive the following edits:
- Edit "02282" – NCPDP code "75", PA Required Call Magellan. This is specific to topical compounds.
- Edit "02283" – NCPDP code "E2", Missing Invalid (M/I) Route of Administration. NCPDP field 995-E2 (Route of Administration) will be required for any topical compound claim.
To obtain a PA, the prescriber must contact the PA clinical call center at (877)309-9493. The PA clinical call center is available 24 hours per day, seven days per week with pharmacy technicians and pharmacists who will work with prescribers or the prescriber agent to quickly obtain a PA.
Questions and Additional Information:
- The 503A FDA bulk agent list can be found on the FDA "Bulk Drug Substances Used in Compounding Under Section 503A of the FD&C Act" web page.
- Medicaid Update articles regarding compounding and other topics can be found on the NYS DOH Medicaid Update homepage.
- A list of reimbursable NYS Medicaid drugs can be found on the eMedNY "Medicaid Pharmacy List of Reimbursable Drugs" web page.
- A list of drugs in the NYRx Pharmacy Programs can be found in the NYRx, the Medicaid Pharmacy Program Preferred Drug List.
- Questions regarding compound billing should be directed to the eMedNY Call Center at (800) 343-9000.
- Questions regarding this policy should be directed to the NYRx by telephone at (518) 486-3209 or by email at NYRx@health.ny.gov.
- Medicaid Managed Care (MMC) enrollee claims, regardless of claim type, pharmacy or medical, are also subject to the requirements above. Any questions, including as to MMC reimbursement, billing, and/or documentation requirements should be directed to the MMC Plan of the enrollee.
NYRx Coverage Policy and Billing Guidance for Pharmacist Dispensing of Self-Administered Hormonal Contraception
Chapter 128 of the Laws of 2023, as amended by Chapter 90 of the Laws of 2024, amends New York State (NYS) Education Law to allow pharmacists licensed and located in NYS to dispense self-administered hormonal contraceptives pursuant to a non-patient specific order written by the Commissioner of Health (COH), a physician-licensed, or a nurse practitioner (NP) certified in NYS.
Self-administered hormonal contraceptives means self-administered contraceptive medications or devices approved by the Food and Drug Administration (FDA) to prevent pregnancy by using hormones to regulate or prevent ovulation, and includes oral hormonal contraceptives, hormonal contraceptive vaginal rings and hormonal contraceptive patches. When used correctly, the medications and devices are 99 percent effective at preventing pregnancy.
The COH is authorized by Public Health Law §267-a to establish a non-patient specific order for pharmacists' dispensing of self-administered hormonal contraception. As described in the standing order, sufficient clinical information should be documented in the profile of the patient when self-administered hormonal contraceptives are dispensed by a state-licensed pharmacist. Pharmacists dispensing self-administered hormonal contraceptives must retain documentation that includes:
- NYS Medicaid member request for services;
- date, time, and duration of the encounter;
- if the NYS Medicaid member is a new or existing patient;
- NYS Medicaid member completed New York State Department of Health CONTRACEPTION: Self-Screening Patient Intake Form (this form documents NYS Medicaid member consent);
- the result of the clinical decision utilizing the New York State Department of Health CONTRACEPTION: Standardized Assessment and Treatment Care Pathway document; and
- if applicable, date and time communication sent to the primary care provider of the individual.
NYRx Pharmacy Billing:
- To bill for the self-administered hormonal contraception, the pharmacy must submit a valid National Drug Code (NDC). Pharmacies must bill the Usual and Customary (U&C) price and will be reimbursed according to NYRx payment methodology and a $10.18 dispensing fee.
- To bill for the evaluation and management (E&M) associated with the dispensing of self-administered hormonal contraception, pharmacies will submit using the National Council for Prescription Drug Programs (NCPDP) D.0 claim format and enter one of the Healthcare Common Procedure Coding System (HCPCS) codes identified below.
- NYRx members may receive up to 12 months of prescription contraceptives at one time for family planning purposes as authorized by Title 18 of the New York Codes, Rules and Regulations (NYCRR) §505.3(e).
- Pharmacies are expected to dispense up to the quantity limit of the prescription or Standing Order, one year supply, at the time of dispensing, pursuant to patient preference or primary insurance coverage limitations.
- Submitting any assessment code more than once a year requires cause.
Billing Instructions for Self-Administered Hormonal Contraception
| NCPDP D.0. Claim Segment Field | Value |
|---|---|
| 436-E1 (Product/Service ID Qualifier) | Enter a value of "03" (NDC). |
| 444-E9 (Pharmacist ID) | Enter pharmacist National Provider Identifier (NPI) number. |
| 411-DB (Prescriber ID) | Enter prescriber NPI number. |
Billing Instructions for E&M Related to Pharmacist Dispensing of Contraception
A state-licensed pharmacist dispensing self-administered hormonal contraceptives may enter one of the following HCPCS codes when performing E&M of a NYRx member:
| HCPCS Code | Value | Fee |
|---|---|---|
| 99605* | Medication therapy management service(s) provided by a pharmacist, individual, face-to-face with patient, with assessment and intervention if provided; 15 minutes, new patient. | $48.20 |
| 99606** | Medication therapy management service(s) provided by a pharmacist, individual, face-to-face with patient, with assessment and intervention if provided; 15 minutes, established patient. | $15.29 |
*A patient would be considered a "new patient" when pharmacist is conducting the initial assessment for self- administered hormonal contraceptives.
**A patient would be considered an "established patient" for subsequent assessments that occur after their initial assessment has been completed.
The above HCPCS codes may be submitted in the NCPDP D.0 format, as outlined below:
| NCPDP D.0. Claim Segment Field | Value |
|---|---|
| 436-E1 (Product/Service ID Qualifier) | Enter the applicable value, which qualifies the code submitted in field 407-D7 (Product/Service ID) as a procedure code. |
| 407-D7 (Product/Service ID) | Enter the value of "99605" if prescribing for a new patient. |
| Enter the value of "99606" if prescribing for an established patient. | |
| 444-E9 (Pharmacist ID) | Enter the pharmacist NPI number. |
| 411-DB (Prescriber ID) | Enter the pharmacy NPI number. |
Providers must refer to the NYRx the NY Medicaid Pharmacy Program – Pharmacy Manual Policy Guidelines, located on the eMedNY "Pharmacy Manual" web page, for further guidance on origin code and serial number values that must be submitted on the claim. Providers may only submit claims for payment for furnished services in which were medically necessary (Title 18 of the NYCRR 504.3(e)). All claims are subject to audit and recovery.
Questions and Additional Information:
- For COH Standing Orders, forms, fact sheets and program information, providers should refer to the NYS DOH "Reproductive Health" web page.
- Pharmacists should refer to the NYSED "Pharmacists" web page, for additional information.
- NYRx billing questions should be directed to the eMedNY Call Center at (800) 343-9000.
- NYRx pharmacy coverage and policy questions should be directed to the Medicaid Pharmacy Policy Unit by telephone at (518) 486-3209 or by email at NYRx@health.ny.gov.
New Medicaid Enrollment Option for Non-Enrolled Providers Seeking Medicare Bad Debt
The Centers for Medicare and Medicaid Services (CMS) amended 42 Code of Federal Regulations (CFR) §455.410(d), to reduce the number of Medicare bad debt appeals. The CMS Final Rule requires Medicare-enrolled non-institutional providers and suppliers seeking to claim Medicare bad debt to enroll in a State's Medicaid program for the sole purpose of receiving a zero-dollar Medicaid Remittance Advice (RA). To comply with the CMS Final Rule, New York State (NYS) Medicaid has implemented two new provider enrollment categories of service (COS).
Background
42 CFR §455.410 was amended to codify Medicare's longstanding "must-bill" policy outlined in 42 CFR §413.89 – Bad debts, charity, and courtesy allowances. In the past, State Medicaid programs have constrained and/or prevented enrollment of certain types of providers or suppliers that are not explicitly included in their State Plan. Medicare-enrolled provider/suppliers that are unable to enroll in a State Medicaid program cannot have their cost-sharing claims adjudicated in the State Medicaid Management Information System (MMIS) nor obtain a Medicaid RA.
This amendment requires the state Medicaid agency to allow enrollments of Medicare-enrolled providers/suppliers meet all federal Medicaid enrollment requirements, including, but not limited to, all applicable provisions of 42 CFR §455, subparts B and E, where such enrollments are solely for the purpose of processing claims to determine Medicare cost-sharing, as defined in §1905(p)(3) of the Social Security Act. This provision does not require States to enroll provider types not recognized by their Medicaid program for purposes other than the submission of Medicare cost-sharing claims, adjudication of such claims, and issuance of a Medicaid RA.
Provider Enrollment
Medicare providers/suppliers who are only seeking a zero-dollar RA to claim Medicare bad debt must choose this Medicare Cost-Sharing Only option for NYS Medicaid enrollment. This includes Medicare-enrolled providers/suppliers who are currently recognized by the NYS Medicaid program (i.e., enrollable, but who wish to engage the NYS Medicaid program for the sole purpose of submitting claims for Medicare cost-sharing to obtain a zero-dollar RA).
Providers interested in enrolling under one of the newly created COS should visit the relevant link below for additional information:
- Medicare-enrolled business/group providers or suppliers (COS "0630"): eMedNY "Provider Enrollment and Maintenance – Medicare Cost Sharing business" web page.
- Medicare-enrolled individual practitioners (COS "0631"): eMedNY "Provider Enrollment and Maintenance – Medicare Cost Sharing Practitioner" web page.
Please note: Billing, rendering/attending, and ordering/referring providers who are reported on Medicare cost-sharing-only claim submissions must also be enrolled in Medicaid under either a Medicare Cost-Sharing Only COS or as a Medicaid provider under a COS already recognized by the State plan.
Claim Submission
Claims submitted to Medicaid containing a billing provider assigned a Medicare Cost-Sharing Only COS will return a zero liability/zero payment RA back to the billing provider. Medicare must be reported as primary on these claim submissions.
Questions and Additional Resources:
- Fee-for-service (FFS) claims reimbursement, and/or provider enrollment questions should be directed to the eMedNY Call Center by telephone at (800) 343-9000 or by visiting the eMedNY homepage, for additional information.
- FFS coverage and policy questions should be directed to the Office of Health Insurance Programs (OHIP) Division of Program Development and Management (DPDM) by telephone at (518) 473-2160 or by email at FFSMedicaidPolicy@health.ny.gov.
The Medicaid Update is a monthly publication of the New York State Department of Health.
Kathy Hochul
Governor
State of New York
James McDonald, M.D., M.P.H.
Acting Commissioner
New York State Department of Health
Amir Bassiri
Medicaid Director
Office of Health Insurance Programs